ORKAMBI (lumacaftor/ivacaftor)
Self-Administration - oral tablet; oral granule packet
Indications for Prior Authorization:
- Cystic Fibrosis: Treatment of cystic fibrosis (CF) in patients 12 years and older who are homozygous for the F508del mutation in the CFTR gene. If the patient's genotype is unknown, an FDA-cleared CF mutation test should be used to detect the presence of the F508del mutation on both alleles of the CFTR gene.
- Limitations of use: Efficacy and safety have not been established in patients with CF other than those homozygous for the F508del mutation.
Coverage Criteria:
For treatment of Cystic Fibrosis (CF):
- Documented diagnosis of CF; AND
- One of the following:
- Request for Orkambi 100mg-125mg tablet: Patient is 6 years of age or older
- Request for Orkambi 200mg-125mg tablet: Patient is 12 years of age or older
- Request for Orkambi granule packet: One of the following:
- Patient is 1 – 5 years of age
- Patient is 6 years of age or older and is unable to swallow oral tablets; AND
- Prescribed by or in consultation with one of the following:
- Specialist affiliated with a cystic fibrosis care center
- Pulmonologist; AND
- Patient is homozygous for the F508del mutation in the CF transmembrane conductance regulator (CFTR) gene as detected by an FDA-cleared cystic fibrosis mutation test or Clinical Laboratory Improvement Amendments (CLIA)-approved facility.
Reauthorization Criteria:
For treatment of CF:
- Documentation of positive clinical response to therapy (i.e., improvement in lung function [forced expiratory volume in one second {FEV1}], decreased number of pulmonary exacerbations; AND
- One of the following:
- Request for Orkambi 100mg-125mg tablet: Patient is 6 years of age or older
- Request for Orkambi 200mg-125mg tablet: Patient is 12 years of age or older
- Request for Orkambi granule packet: One of the following:
- Patient is 1 – 5 years of age
- Patient is 6 years of age or older and is unable to swallow oral tablets.
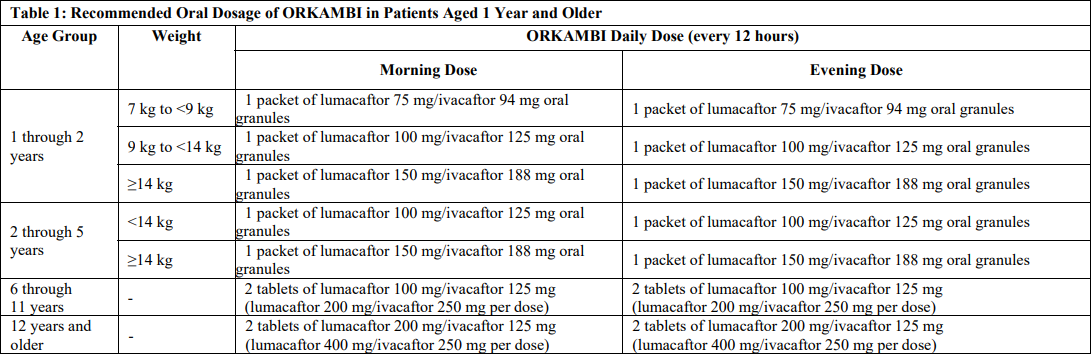
Dosing
- CF:
Coverage Duration:
- 1 year
Authorization is not covered for the following:
- The use of this drug for indications not listed in this policy does not meet the coverage criteria established by the Western Health Advantage (WHA) Pharmacy and Therapeutics (P&T) Committee.
Additional Information:
- Adjust dosing for patients with hepatic impairment or taking CYP3A inhibitors. See the package labeling for additional information.
- Administration Instructions for ORKAMBI Oral Granules: The entire content of each packet of oral granules should be mixed with one teaspoon (5 mL) of age-appropriate soft food or liquid and the mixture completely consumed. Some examples of soft foods or liquids include puréed fruits or vegetables, flavored yogurt or pudding, applesauce, water, milk, breast milk, infant formula or juice. Food should be at room temperature or below. Each packet is for single use only. Once mixed, the product has been shown to be stable for one hour, and therefore should be ingested during this period.
- Administration with Fat-Containing Food for ORKAMBI Tablets and Oral Granules: A fat-containing meal or snack should be consumed just before or just after dosing for all formulations. Examples of appropriate fat-containing foods include eggs, avocados, nuts, butter, peanut butter, cheese pizza, breast milk, infant formula, whole-milk dairy products (such as whole milk, cheese, and yogurt), etc.
- Missed Dose: If a patient misses a dose and remembers the missed dose within 6 hours, the patient should take the dose with fat-containing food. If more than 6 hours elapsed after the usual dosing time, the patient should skip that dose and resume the normal schedule for the following dose. A double dose should not be taken to make up for the forgotten dose.
Review History:
- 07/24/2016 – New utilization management program for established drug approved by P&T.
- 11/14/2023 – Added Orkambi 75mg-94mg granule packet to guideline. Changed age criterion where appropriate to align with PI. Added reauthorization criteria. Updated dosing, background and references. (P&T 11/14/2023)
References:
- Orkambi Prescribing Information. Vertex Pharmaceuticals Incorporated. Boston, MA. September 2022.
Last review date: November 14, 2023