Nivestym (filgrastim-aafi)*; Zarxio (filgrastim-sndz)*; Neupogen (filgrastim); Granix (tbo-filgrastim); Releuko (filgrastim-ayow)
Office-Administration or Self-Administration
* preferred filgrastim agents for pharmacy benefit only
Diagnosis considered for coverage:
- Febrile Neutropenia (FN), Prophylaxis – Indicated to reduce the duration of severe neutropenia in adult and pediatric patients 1 month and older with nonmyeloid malignancies receiving myelosuppressive anticancer drugs associated with a clinically significant incidence of febrile neutropenia. (Granix)
- Febrile Neutropenia (FN), Prophylaxis – Indicated to decrease the incidence of infection, as manifested by FN, in patients with nonmyeloid malignancies receiving myelosuppressive anti-cancer drugs associated with a significant incidence of severe neutropenia with fever. (Neupogen, Zarxio, Nivestym, Releuko)
- Patients with Acute Myeloid Leukemia (AML) Receiving Induction or Consolidation Chemotherapy – Indicated for reducing the time to neutrophil recovery and the duration of fever, following induction or consolidation chemotherapy treatment of adults with AML. (Neupogen, Zarxio, Nivestym, Releuko)
- Patients with Cancer Undergoing Bone Marrow Transplantation (BMT) – Indicated to reduce the duration of neutropenia and neutropenia-related clinical sequelae, e.g., febrile neutropenia, in patients with nonmyeloid malignancies undergoing myeloablative chemotherapy followed by bone marrow transplantation. (Neupogen, Zarxio, Nivestym, Releuko)
- Patients Undergoing Autologous Peripheral Blood Progenitor Cell (PBPC) Collection and Therapy – Indicated for the mobilization of autologous hematopoietic progenitor cells into the peripheral blood for collection by leukapheresis. (Neupogen, Zarxio, Nivestym)
- Patients with Severe Chronic Neutropenia (SCN) – Indicated for chronic administration to reduce the incidence and duration of sequelae of neutropenia (e.g., fever, infections, oropharyngeal ulcers) in symptomatic patients with congenital neutropenia, cyclic neutropenia, or idiopathic neutropenia. (Neupogen, Zarxio, Nivestym, Releuko)
- Hematopoietic Syndrome of Acute Radiation Syndrome (H-ARS) – Indicated to increase survival in patients acutely exposed to myelosuppressive doses of radiation. (Neupogen)
Off Label Uses: - Treatment of High-Risk Febrile Neutropenia (FN) – For the treatment of FN in patients who have received or are receiving myelosuppressive anticancer drugs associated with neutropenia who are at high risk for infection-associated complications. (Granix, Neupogen, Zarxio, Nivestym, Releuko)
- Hematopoietic Subsyndrome of Acute Radiation Syndrome – To increase survival in patients acutely exposed to myelosuppressive doses of radiation. (Granix, Zarxio, Nivestym, Releuko)
- Human Immunodeficiency Virus (HIV)-Related Neutropenia – Has been prescribed for HIV-related neutropenia. (Neupogen, Zarxio, Nivestym, Releuko)
- Hepatitis-C Interferon Induced Neutropenia – Has been prescribed for interferon-induced neutropenia in Hepatitis C virus infected patients. (Neupogen, Zarxio, Nivestym, Releuko)
- Patients Undergoing Autologous Peripheral Blood Progenitor Cell (PBPC) Collection and Therapy – For the mobilization of autologous hematopoietic progenitor cells into the peripheral blood for collection by leukapheresis. (Granix, Releuko)
- Myelodysplastic syndrome (MDS)-related neutropenia – Has been prescribed for patients with MDS-related neutropenia. (Granix, Neupogen, Nivestym, Releuko, Zarxio)
Coverage Criteria:
For prophylaxis of febrile neutropenia (FN) or treatment of FN (off-label) caused by myelosuppressive chemotherapy and request for Granix, Neupogen, Zarxio, Nivestym, or Releuko:
- Dose does not exceed recommended maximum:
- Granix: 5 mcg/kg/day for up to 14 days per chemotherapy cycle
- Neupogen, Zarxio, Nivestym, Releuko: 10 mcg/kg/day for up to 14 days per chemotherapy cycle; AND
- Prescribed by or in consultation with a hematologist or oncologist; AND
- Medical records document ONE of the following:
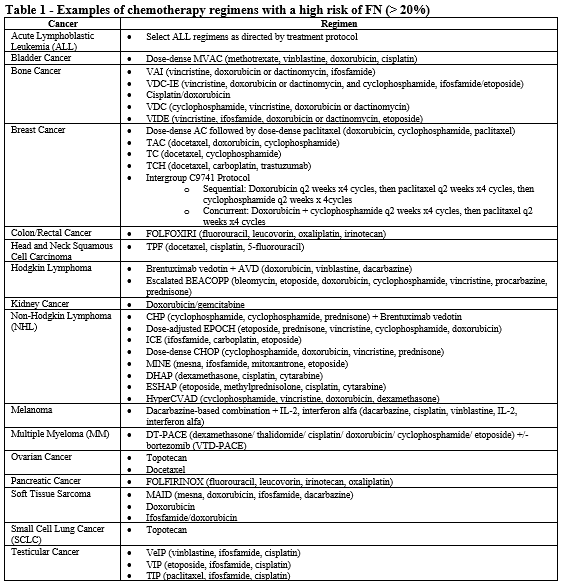
- Patient is receiving chemotherapy regimen(s) associated with greater than 20% incidence of FN (see Table 1).
- All of the following:
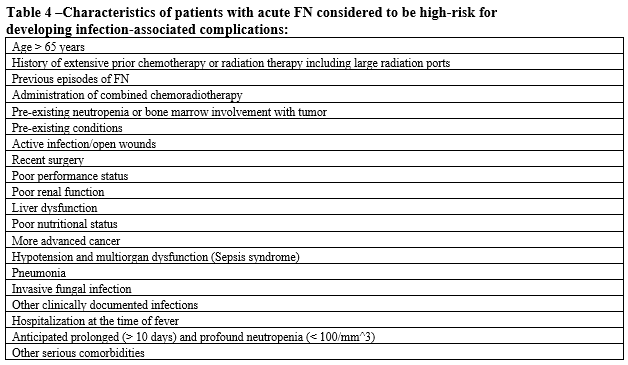
- Diagnosis of acute febrile neutropenia (FN).
- Patient is at high risk for infection-associated complications (see Table 4).
- Absolute neutrophil count (ANC) is less than 1000 cells/mm3.
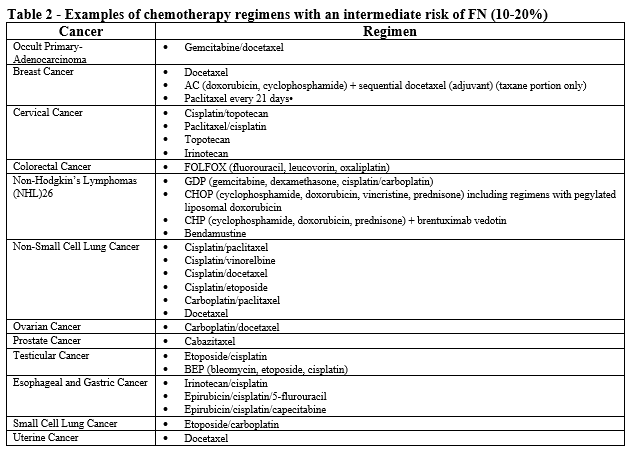
- Both of the following:
- Patient is receiving chemotherapy regimen(s) associated with 10-20% incidence of FN (see Table 2).
- Patient has one or more risk factors associated with chemotherapy induced infection, FN, or neutropenia.
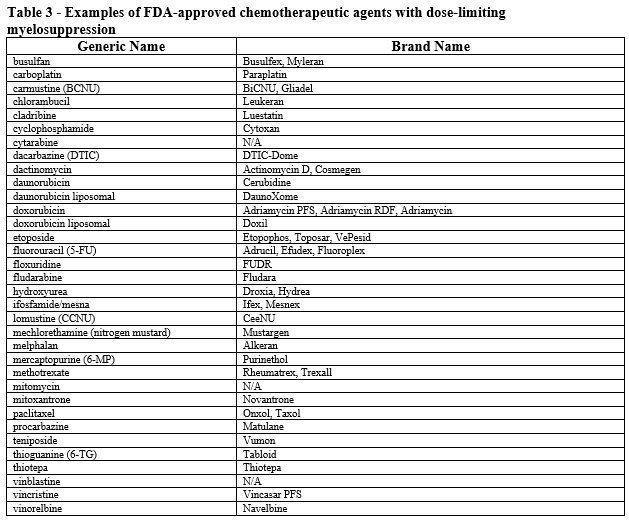
- Both of the following:
- Patient is receiving myelosuppressive anticancer drugs associated with neutropenia (see Table 3).
- Patient has a history of FN or dose-limiting event during a previous course of chemotherapy (i.e., secondary prophylaxis); AND
- Requested G-CSF agent will not be used in combination with other G-CSF/GM-CSF agents; AND
- One of the following:
- For requests prescribed through the medical benefit: no product preference
- For requests prescribed through the pharmacy benefit:
- For Granix, Neupogen, or Releuko only – Tried and failed, contraindicated, or intolerant to BOTH of the following:
- Nivestym
- Zarxio
- For Granix, Neupogen, or Releuko only – Tried and failed, contraindicated, or intolerant to BOTH of the following:
For prevention or reduction of neutropenia associated with acute myeloid leukemia (AML) induction or consolidation chemotherapy and request for Neupogen, Zarxio, Nivestym, or Releuko:
- Dose does not exceed 10 mcg/kg/day for up to 14 days per chemotherapy cycle; AND
- Prescribed by or in consultation with a hematologist or oncologist; AND
- Medical records document the patient is receiving induction or consolidation chemotherapy for AML; AND
- Requested G-CSF agent will not be used in combination with other G-CSF/GM-CSF agents; AND
- One of the following:
- For requests prescribed through the medical benefit: no product preference
- For requests prescribed through the pharmacy benefit:
- For Neupogen and Releuko only – Tried and failed, contraindicated, or intolerant to BOTH of the following:
- Nivestym
- Zarxio
- For Neupogen and Releuko only – Tried and failed, contraindicated, or intolerant to BOTH of the following:
For stem cell mobilization or bone marrow transplant (BMT) and request for Granix, Neupogen, Zarxio, Nivestym, or Releuko:
- Dose does not exceed 10 mcg/kg/day; AND
- Prescribed by or in consultation with a hematologist or oncologist; AND
- Medical records document ONE of the following:
- Used for the mobilization of hematopoietic progenitor cells into the peripheral blood for collection by leukapheresis.
- Patient has had a peripheral stem cell transplant (PSCT) treated with myeloablative chemotherapy.
- Patient has a non-myeloid malignancy treated with myeloablative chemotherapy followed by allogeneic or autologous BMT; AND
- Requested G-CSF agent will not be used in combination with other G-CSF/GM-CSF agents; AND
- One of the following:
- For requests prescribed through the medical benefit: no product preference
- For requests prescribed through the pharmacy benefit:
- For Granix, Neupogen, or Releuko only – Tried and failed, contraindicated, or intolerant to BOTH of the following:
- Nivestym
- Zarxio
- For Granix, Neupogen, or Releuko only – Tried and failed, contraindicated, or intolerant to BOTH of the following:
For diagnosis of severe chronic neutropenia (SCN) (i.e., congenital, cyclic, or idiopathic neutropenia; agranulocytosis) and request for Neupogen, Nivestym, Releuko, or Zarxio:
- Starting dose does not exceed:
- Congenital Neutropenia: 6 mcg/kg twice daily
- Idiopathic or Cyclic Neutropenia: 5 mcg/kg once daily
- Subsequent doses are individualized based on the patient’s clinical course as well as ANC; AND
- Prescribed by or in consultation with a hematologist or oncologist; AND
- Medical records document SCN with a history of absolute neutrophil count (ANC) less than or equal to 500 cells/mm3; AND
- Medical records document one of the following:
- Patient has had multiple clinically significant infections requiring treatment with antibiotics within the past year.
- Patient has been hospitalized for a clinically significant infection requiring IV antibiotics within the past year; AND
- Requested G-CSF agent will not be used in combination with other G-CSF/GM-CSF agents; AND
- One of the following:
- For requests prescribed through the medical benefit: no product preference
- For requests prescribed through the pharmacy benefit:
- For Neupogen and Releuko only – Tried and failed, contraindicated, or intolerant to BOTH of the following:
- Nivestym
- Zarxio
- For Neupogen and Releuko only – Tried and failed, contraindicated, or intolerant to BOTH of the following:
For prevention or treatment of acute radiation syndrome (ARS) neutropenia and request for Neupogen, Nivestym (off-label), Releuko (off-label), or Zarxio (off-label):
- Dose does not exceed 10 mcg/kg/day; AND
- Prescribed by or in consultation with a radiologist, hematologist or oncologist; AND
- Medical records document the patient was, or will be, exposed to myelosuppressive doses of radiation; AND
- Requested G-CSF agent will not be used in combination with other G-CSF/GM-CSF agents; AND
- One of the following:
- For requests prescribed through the medical benefit: no product preference
- For requests prescribed through the pharmacy benefit:
- For Neupogen and Releuko only – Tried and failed, contraindicated, or intolerant to BOTH of the following:
- Nivestym
- Zarxio
- For Neupogen and Releuko only – Tried and failed, contraindicated, or intolerant to BOTH of the following:
For diagnosis of human immunodeficiency virus (HIV)-related neutropenia and request for Neupogen (off-label), Nivestym (off-label), Releuko (off-label), or Zarxio (off-label):
- Dose does not exceed the recommended dose of 10 mcg/kg per day; AND
- Prescribed by or in consultation with a hematologist, oncologist, or infectious disease specialist; AND
- Patient has documented HIV with absolute neutrophil count (ANC) less than or equal to 1,000 cells/mm3; AND
- Requested G-CSF agent will not be used in combination with other G-CSF/GM-CSF agents; AND
- One of the following:
- For requests prescribed through the medical benefit: no product preference
- For requests prescribed through the pharmacy benefit:
- For Neupogen and Releuko only – Tried and failed, contraindicated, or intolerant to BOTH of the following:
- Nivestym
- Zarxio
- For Neupogen and Releuko only – Tried and failed, contraindicated, or intolerant to BOTH of the following:
For diagnosis of peginterferon-related neutropenia and request for Neupogen (off-label), Nivestym (off-label), Releuko (off-label), or Zarxio (off-label):
- Dose does not exceed recommended FDA-maximum of 10 mcg/kg per day; AND
- Prescribed by or in consultation with a hematologist, oncologist, infectious disease specialist, hepatologist, or gastroenterologist; AND
- Patient is undergoing treatment with Peg-Intron (peginterferon alfa-2b) or Pegasys (peginterferon alfa-2a); AND
- One of the following is supported by medical record documentation:
- Patient has documented hepatitis C virus (HCV) and ANC is less than or equal to 500 cells/mm3 after a dose reduction of peginterferon (i.e., 135 mcg or less per week).
- Patient has a co-infection with human immunodeficiency virus (HIV):
- Patient is status post liver transplant
- Patient has established cirrhosis (fibrosis score of F4); AND
- Requested G-CSF agent will not be used in combination with other G-CSF/GM-CSF agents; AND
- One of the following:
- For requests prescribed through the medical benefit: no product preference
- For requests prescribed through the pharmacy benefit:
- For Neupogen and Releuko only – Tried and failed, contraindicated, or intolerant to BOTH of the following:
- Nivestym
- Zarxio
- For Neupogen and Releuko only – Tried and failed, contraindicated, or intolerant to BOTH of the following:
For diagnosis of myelodysplastic syndrome (MDS)-related neutropenia (off-label) and request for Granix (off-label), Neupogen (off-label), Nivestym (off-label), Releuko (off-label), or Zarxio (off-label):
- Dose does not exceed recommended maximum 1-2 mcg/kg twice weekly; AND
- Prescribed by or in consultation with a hematologist, oncologist; AND
- Medical records document patient has MDS; AND
- Absolute neutrophil count (ANC) less than or equal to 500 cells/mm3; AND
- Medical records document one of the following:
- Patient has had multiple clinically significant infections requiring treatment with antibiotics within the past year.
- Patient has been hospitalized for a clinically significant infection requiring IV antibiotics within the past year; AND
- Requested G-CSF agent will not be used in combination with other G-CSF/GM-CSF agents; AND
- One of the following:
- For requests prescribed through the medical benefit: no product preference
- For requests prescribed through the pharmacy benefit:
- For Granix, Releuko, or Neupogen only – Tried and failed, contraindicated, or intolerant to BOTH of the following:
- Nivestym
- Zarxio
- For Granix, Releuko, or Neupogen only – Tried and failed, contraindicated, or intolerant to BOTH of the following:
Reauthorization Criteria:
For prophylaxis of febrile neutropenia (FN) or treatment of FN (off-label) caused by myelosuppressive chemotherapy and request for Granix, Neupogen, Zarxio, Nivestym, or Releuko:
- Dose does not exceed recommended maximum:
- Granix: 5 mcg/kg/day for up to 14 days per chemotherapy cycle
- Neupogen, Zarxio, Nivestym, Releuko: 10 mcg/kg/day for up to 14 days per chemotherapy cycle; AND
- Medical records document the patient continues to receive myelosuppressive chemotherapy; AND
- Requested G-CSF agent will not be used in combination with other G-CSF/GM-CSF agents.
For diagnosis of severe chronic neutropenia (SCN) (i.e., congenital, cyclic, or idiopathic neutropenia; agranulocytosis) and request for Neupogen, Nivestym, Releuko, or Zarxio:
- Dose has been individualized based on the patient’s clinical course as well as ANC; AND
- Medical records document the patient’s absolute neutrophil count (ANC) has stabilized while on G-CSF therapy; AND
- Requested G-CSF agent will not be used in combination with other G-CSF/GM-CSF agents.
For diagnosis of human immunodeficiency virus (HIV)-related neutropenia and request for Neupogen (off-label), Nivestym (off-label), Releuko (off-label), or Zarxio (off-label):
- Dose does not exceed the recommended dose of 10 mcg/kg per day; AND
- Medical records document the patient’s absolute neutrophil count (ANC) has stabilized while on G-CSF therapy; AND
- Requested G-CSF agent will not be used in combination with other G-CSF/GM-CSF agents.
For diagnosis of myelodysplastic syndrome (MDS)-related neutropenia (off-label) and request for Granix (off-label), Neupogen (off-label), Nivestym (off-label), Releuko (off-label), or Zarxio (off-label):
- Dose does not exceed recommended maximum 1-2 mcg/kg twice weekly; AND
- Medical records document the patient’s absolute neutrophil count (ANC) has stabilized while on G-CSF therapy; AND
- Requested G-CSF agent will not be used in combination with other G-CSF/GM-CSF agents.
Coverage Duration:
-
FN prophylaxis or high-risk FN treatment
-
Initial: 3 months
-
Reauthorization: duration of chemotherapy
-
-
AML: 2 months
-
BMT: 2 months
-
SCN
-
Initial: 6 months
-
Reauthorization: 1 year
-
-
ARS: 1 month
-
HIV-related neutropenia
-
Initial: 6 months
-
Reauthorization: 6 months
-
-
PEG-related neutropenia: same end date as peginterferon authorization
-
MDS-related neutropenia
-
Initial: 3 months
-
Reauthorization: 6 months
-
Authorization is not covered for the following:
- The use of this drug for indications not listed in this policy does not meet the coverage criteria established by the Western Health Advantage (WHA) Pharmacy and Therapeutics (P&T) Committee.
Additional Information:
- The FDA defines biosimilar as a biological product that is highly similar to and has no clinically meaningful differences from an existing FDA-approved reference product. The American Society of Clinical Oncology states that pegfilgrastim, filgrastim, tbo-filgrastim, and filgrastim-sndz (and other biosimilars as they become available) can be used for the prevention of treatment-related febrile neutropenia. The choice of agent depends on convenience, cost, and clinical situation. NCCN lists FDA-approved biosimilars as appropriate substitutes for filgrastim and pegfilgrastim. Limited data suggest that patients can alternate between the biosimilar and the originator biologic without any clinically meaningful differences regarding efficacy or safety.
- Dose dense chemotherapy is a treatment plan in which drugs are given with less time between treatments than in a standard chemotherapy treatment plan.
- FN is described by clinical practice guidelines as neutropenia with a single oral or tympanic temperature greater than or equal to 101°F (38.3°C) or greater than or equal to 100.4°F (38°C) for at least one hour.
- The Infectious Diseases Society of America (IDSA) guidelines recommend against their use for all patients with established fever and neutropenia, whereas the American Society of Clinical Oncology and National Comprehensive Cancer Network guidelines state that their use can be "considered" for patients at high risk for infection-associated complications or who have prognostic factors that are predictive of a poor clinical outcome.
- There are no studied that have addressed therapeutic use of filgrastim for febrile neutropenia in patients who have already received prophylactic pegfilgrastim. However pharmacokinetic data of pegfilgrastim demonstrated high levels during neutropenia and suggest that additional G-CSFs may not be beneficial; however, in patients with prolonged neutropenia additional G-CSFs may be considered.
- Non-myeloid malignancies are cancers that are not classified as: (1) myelodysplastic syndromes (MDSs); (2) myeloproliferative neoplasms (MPNs); (3) myelodysplastic/myeloproliferative neoplasms (MDS/MPN); or (4) myeloid malignancies associated with eosinophilia and abnormalities of growth factor receptors derived from platelets or fibroblasts.
- Bone marrow transplants are most commonly used in the following diseases: leukemias, lymphomas, multiple myeloma, immune deficiency disorders, severe aplastic anemia, and some solid-tumor cancers (in rare circumstances).
- Hematopoietic progenitor cells (HPCs) or hematopoietic stem cells (HSCs) are cells present in blood and bone marrow. HPCs are capable of forming mature blood cells, such as red blood cells (the cells that carry oxygen), platelets (the cells that help stop bleeding) and white blood cells (the cells that fight infections). Hematopoietic stem cell transplantation is the IV infusion of hematopoietic stem and progenitor cells designed to establish marrow and immune function in patients with a variety of acquired and inherited malignant and nonmalignant disorders.
- Allogeneic stem cell transplantation involves transferring the stem cells from a healthy person (the donor) to the patient’s body after high-intensity chemotherapy or radiation. The donated stem cells can come from either a related or an unrelated donor.
- An autologous stem cell transplant uses healthy blood stem cells from your own body to replace your diseased or damaged bone marrow. An autologous stem cell transplant is also called an autologous bone marrow transplant.
-
One cubic millimeter (mm3) = One microliter (µL).
-
Meters squared (m2) is the unit used to describe body surface area (BSA). The calculation is derived from height and weight measurements. A calculator to determine BSA can be found at http://www.medcalc.com/body.html or https://www.calculator.net/body-surface-area-calculator.html
Review History:
- 07/24/2016 - Previous review
- 02/15/2022 - P&T review of filgrastim drug class; separation of filgrastim policy from other myeloid colony stimulating factors (i.e. pegfilgrastim agents, Leukine); new criteria created including addition of Granix and all FDA-approved filgrastim biosimilar agents to policy; update dosing guidance based on package inserts and drug compendia; added coverage criteria for FDA-approved indications and off-label uses; add off-label coverage criteria for MDS, update coverage durations; add preferred agents under pharmacy benefit only if clinically appropriate.
- 12/1/2022- Addition of Releuko to filgrastim criteria as a non-preferred product. Updated dosing for SCN and MDS
References:
- Aarts MJ, Peters FP, Mandigers CM, et al. Primary granulocyte colony-stimulating factor prophylaxis during the first two cycles only or throughout all chemotherapy cycles in patients with breast cancer at risk for febrile neutropenia. J Clin Oncol. 2013;31:4290-4296.
- Arora M, Burns LJ, Barker JN, et al. Randomized comparison of granulocyte colony-stimulating factor versus granulocyte-macrophage colony-stimulating factor plus intensive chemotherapy for peripheral blood stem cell mobilization and autologous transplantation in multiple myeloma. Biol Blood Marrow Transplant. 2004;10(6):395-404.
- Blackwell K, Semiglazov V, Krasnozhon D, et al. Comparison of EP2006, a filgrastim biosimilar, to the reference: a phase III, randomized, double-blind clinical study in the prevention of severe neutropenia in patients with breast cancer receiving myelosuppressive chemotherapy. Ann Oncol. 2015;26:1948-1953.
- Blackwell K, Donskih R, Jones CM, et al. A comparison of proposed biosimilar LA-EP2006 and reference pegfilgrastim for the prevention of neutropenia in patients with early-stage breast cancer receiving myelosuppressive adjuvant or neoadjuvant chemotherapy: Pegfilgrastim Randomized Oncology (Supportive Care) Trial to Evaluate Comparative Treatment (PROTECT-2), a phase III, randomized, double-blind trial. Oncologist. 2016;21(7):789-794. doi: 10.1634/theoncologist.2016-0011.
- Bohlius J, Herbst C, Reiser M, et al. Granulopoiesis-stimulating factors to prevent adverse effects in the treatment of malignant lymphoma. Cochrane Database Syst Rev. 2008 Oct 8;(4):CD003189.
- Botteri E, Krendyukov A, Curigliano G. Comparing granulocyte colony-stimulating factor filgrastim and pegfilgrastim to its biosimilars in terms of efficacy and safety: a meta analysis of randomized clinical trials in breast cancer patients. Eur J Cancer.2018;89:49-55
- Cooper KL, Madan J, Whyte S, et al. Granulocyte colony-stimulating factors for febrile neutropenia prophylaxis following chemotherapy: systematic review and meta-analysis. BMC Cancer. 2011 Sep 23; 11:404.
- Crawford J, Caserta C, Roila F, on behalf of the ESMO Guidelines Working Group. Hematopoietic growth factors: ESMO clinical practice guidelines for the applications. Ann Oncol. 2010;21(suppl 5): v248-v251.
- Dale DC, Bonilla MA, Davis MW, et al. A randomized controlled phase III trial of recombinant human granulocyte colony-stimulating factor (filgrastim) for treatment of severe chronic neutropenia. Blood. 1993;81:2496-2502.
- Duong HK, Savani BN, Copelan E, et al. Peripheral blood progenitor cell mobilization for autologous and allogeneic hematopoietic cell transplantation: guidelines from the American Society for Blood and Marrow Transplantation. Biol Blood Marrow Transplant. 2014;20:1262-1273.
- Engert A, Griskevicius L, Zyuzgin Y, et al. XM02, the first granulocyte colony-stimulating factor biosimilar, is safe and effective in reducing the duration of severe neutropenia and incidence of febrile neutropenia in patients with non-Hodgkin lymphoma receiving chemotherapy. Leuk Lymphoma. 2009;50(3):374-379.
- Gatzemeier U, Ciuleanu T, Dediu M, et al. XM02, the first biosimilar G-CSF, is safe and effective in reducing the duration of severe neutropenia and incidence of febrile neutropenia in patients with small cell or non-small cell lung cancer receiving platinum-based chemotherapy. J Thorac Oncol. 2009;4:736-740.
- Giralt S, Costa L, Schriber J, et al. Optimizing autologous stem cell mobilization strategies to improve patient outcomes: consensus guidelines and recommendations. Biol Blood Marrow Transplant. 2014;20(3): 295-308. doi: 10.1026/j.bbmt.2013.
- Granix [package insert]. Teva Pharmaceuticals USA, Inc. North Wales, PA; April 2020.
- Gurion R, Belnik-Plitman Y, Gafter-Gvili A, et al. Colony-stimulating factors for prevention and treatment of infectious complications in patients with acute myelogenous leukemia. Cochrane Database of Systematic Reviews, 2012, Issue 6. Art. No.: CD008238. doi: 10.1002/14651858.CD008238.pub3.
- Heil G, Hoelzer D, Sanz MA, et al. A randomized, placebo-controlled, phase III study of filgrastim in remission induction and consolidation therapy for adults with de novo acute myeloid leukemia. Blood. 1997;90:4710-4718.
- Kim S, Baek J, Min H. Effects of prophylactic hematopoietic colony stimulating factors on stem cell transplantations: meta-analysis. Arch Pharm Res. 2012;35(11):2013-2020. doi: 10.1007/s12272-012-1119-2.
- Klastersky J, de Naurois J, Rolston K, et al. Management of febrile neutropaenia: ESMO clinical practice guidelines. Ann Oncol. 2016;27(suppl 5):v111-v118.
- Kuan JW, Su AT, Leong CF. Pegylated granulocyte-colony stimulating factor versus non-pegylated granulocyte-colony stimulating factor for peripheral blood stem cell mobilization: a systematic review and meta-analysis. J Clin Apher. 2017;32(6):517-542.
- Li X, Zheng H, Yu MC, et al. Is PEGylated G-CSF superior to G-CSF in patients with breast cancer receiving chemotherapy? A systematic review and meta-analysis. Support Care Cancer. 2020;28(11):5085-5097. doi: 10.1007/s00520-020-05603-w.
- Lucas AJ, Olin JL, Coleman MD. Management and preventive measures for febrile neutropenia. Pharmacy and Therapeutics. 2018 Apr;43(4):228.
- Martino M, Praticò G, Messina G, et al. Pegfilgrastim compared to filgrastim after high-dose melphalan and autologous hematopoietic peripheral blood stem cell transplantation in multiple myeloma patients. Eur J Haematol. 2006 Nov;77(5):410-415.
- Mitchell S, Li X, Woods M, et al. Comparative effectiveness of granulocyte colony-stimulating factors to prevent febrile neutropenia and related complications in cancer patients in clinical practice: a systematic review. J Oncol Pharm Practice. 2016;22(5):702-716.
- National Comprehensive Cancer Network (NCCN) clinical practice guidelines in oncology: hematopoietic growth factors. Version 1.2022. NCCN website. https://www.nccn.org/professionals/physician_gls/pdf/growthfactors.pdf. Updated December 22, 2021. Accessed February 7, 2022.
- Nemunaitis J, Rabinowe SN, Singer JW, et al. Recombinant granulocyte-macrophage colony-stimulating factor after autologous bone marrow transplantation for lymphoid cancer. N Engl J Med. 1991;324(25):1773-1778.
- Neupogen [package insert], Amgen Inc. Thousand Oaks, CA; February 2021.
- Nivestym [package insert]. Pfizer Laboratories. Lake Forest, IL; April 2021.
- Page AV, Liles WC. Immunomodulators. In: Bennett JE, Dolin R, Blaser MJ, editors. Mandell, Douglas, and Bennett’s Principles and Practice of Infectious Diseases. 9th ed. Philadelphia: Elsevier: 2020.
- Passweg JR, Baldomero H, Gratwohl A, et al. The EBMT activity survey: 1990-2010. Bone Marrow Transplant. 2012;47:906-923.
- Paul M, Ram R, Kugler E, et al. Subcutaneous versus intravenous granulocyte colony stimulating factor for the treatment of neutropenia in hospitalized hemato-oncological patients: randomized controlled trial. Am J Hematol. 2014. 89(3):243-248.
- Rabinowe SN, Neuberg D, Bierman PJ, et al. Long-term follow-up of a phase III study of recombinant human granulocyte-macrophage colony-stimulating factor after autologous bone marrow transplantation for lymphoid malignancies. Blood. 1993 Apr 1;81(7):1903-1908.
- Renner P, Milazzo S, Liu JP, et al. Primary prophylactic colony-stimulating factors for the prevention of chemotherapy-induced febrile neutropenia in breast cancer patients. Cochrane Database of Systematic Reviews, 2012, Issue 10. Art. No.; CD007913. doi: 10.1002/14651858.CD007913.pub2.
- Rifkin R, Spitzer G, Orloff G, et al. Pegfilgrastim appears equivalent to daily dosing of filgrastim to treat neutropenia after autologous peripheral blood stem cell transplantation in patients with Non-Hodgkin Lymphoma. Clin Lymphoma Myeloma Leuk. 2010;10:186-191.
- Rowe JM, Andersen JW, Mazza JJ, et al. A randomized placebo-controlled phase III study of granulocyte-macrophage colony-stimulating factor in adult patients (> 55 to 70 years of age) with acute myelogenous leukemia: a study of the Eastern Cooperative Oncology Group (E1490). Blood. 1995 Jul 15;86(2):457-462.
- Last review date: December 1, 2022

