VESICARE LS (solifenacin succinate suspension)
Self-Administration - Oral
Indications for Prior Authorization:
- Indicated for the treatment of neurogenic detrusor overactivity (NDO) in pediatric patients aged 2 years and older
Coverage Criteria:
For diagnosis of neurogenic detrusor overactivity:
- Dose is appropriate for the patient’s weight OR dose does not exceed 10 mL (10 mg) once daily, AND
- Patient is 2 years of age or older, AND
- Medical records document a diagnosis of neurogenic detrusor overactivity (NDO), AND
- Patient has tried and failed (a minimum 30-day supply), contraindication (e.g., safety concerns, not indicated for patient's age/weight), or intolerance to generic oxybutynin syrup or tablets
Reauthorization Criteria:
For diagnosis of neurogenic detrusor overactivity:
- Dose is appropriate for the patient’s weight OR dose does not exceed 10 mL (10 mg) once daily, AND
- Medical records document a positive response to therapy
Coverage Duration:
- Initial: 1 year
- Reauthorization: 1 year
Authorization is not covered for the following:
The use of this drug for indications not listed in this policy does not meet the coverage criteria established by the Western Health Advantage (WHA) Pharmacy and Therapeutics (P&T) Committee.
Additional Information:
- The recommended doses are weight-based and are administered once daily
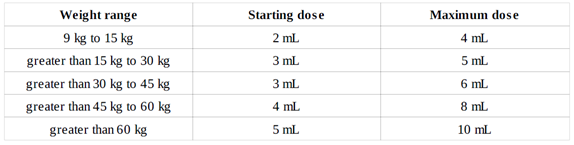
- Do not exceed the recommended Vesicare LS oral suspension starting dose in patients with severe renal impairment (CL < 30 mL/min/1.73 m2)
- Do not exceed the recommended Vesicare LS oral suspension starting dose in patients with moderate hepatic impairment (Child-Pugh B). Do not use Vesicare LS in patients with severe hepatic impairment (Child-Pugh C)
- Do not exceed the recommended Vesicare LS oral suspension starting dose when Vesicare LS is administered with strong CYP3A4 inhibitors such as ketoconazole
- Contraindications
- With gastric retention
- With uncontrolled narrow-angle glaucoma and
- Who have demonstrated hypersensitivity to solifenacin succinate or the inactive ingredients in Vesicare LS oral suspension. Reported adverse reactions have included anaphylaxis and angioedema
- Warnings: angioedema and anaphylactic reactions, urinary retention, gastrointestinal disorders, CNS effects, controlled narrow-angle glaucoma, and QT prolongation in patients at high risk of QT prolongation
Policy Updates:
- 11/16/2021 – New policy approved by P&T
References:
- Vesicare LS Prescribing Information. Astellas Pharma US, Inc. Northbrook, IL. June 2020.
Last review date: November 16, 2021

