KALYDECO (ivacaftor)
Self-Administration - Oral
Indications for Prior Authorization:
- Indicated for the treatment of cystic fibrosis (CF) in patients age 4 months and older who have one mutation in the CFTR gene that is responsive to ivacaftor potentiation based on clinical and/or in vitro assay data
- If the patient's genotype is unknown, an FDA-cleared CF mutation test should be used to detect the presence of a CFTR mutation followed by verification with bi-directional sequencing when recommended by the mutation test instructions for use.
Coverage Criteria:
For diagnosis of cystic fibrosis (CF):
- Dose is appropriate for the patient’s age and weight OR does not exceed 150mg every 12 hours, AND
- Patient is 4 months of age or older, AND
- Patient has a diagnosis of cystic fibrosis (CF) as confirmed by chart note documentation, AND
- Patient has at least one mutation in the cystic fibrosis transmembrane conductance regulator (CFTR) gene that is responsive to ivacaftor potentiation based on clinical and/or in vitro assay data as detected by an FDA-cleared cystic fibrosis mutation test or a test performed at a Clinical Laboratory Improvement Amendments (CLIA)-approved facility, AND
- Prescribed by or in consultation with a pulmonologist or a specialist affiliated with a CF care center, AND
- Kalydeco is not prescribed concurrently with other CFTR modulators (e.g., Orkambi, Symdeko, Trikafta)
Reauthorization Criteria:
For diagnosis of cystic fibrosis (CF):
- Dose is appropriate for the patient’s age and weight OR does not exceed 150mg every 12 hours, AND
- Documentation of positive clinical response (i.e., improvement in lung function [percent predicted forced expiratory volume in one second {PPFEV1}], decreased number of pulmonary exacerbations) to therapy, AND
- Prescribed by or in consultation with a pulmonologist or a specialist affiliated with a CF care center, AND
- Kalydeco is not prescribed concurrently with other CFTR modulators (e.g., Orkambi, Symdeko, Trikafta)
Coverage Duration:
- Initial: 1 year
- Reauthorization: 1 year
Authorization is not covered for the following:
The use of this drug for indications not listed in this policy does not meet the coverage criteria established by the Western Health Advantage (WHA) Pharmacy and Therapeutics (P&T) Committee.
Additional Information:
- Adults and pediatric patients ages 6 years and older: 150 mg every 12 hours
- Patients ages 4 months to less than 6 years:
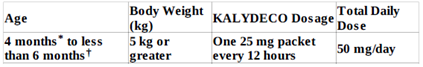
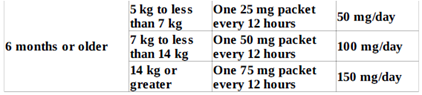
- Patients aged 6 months and older with moderate hepatic impairment (Child-Pugh Class B): one tablet or one packet of oral granules once daily
- Patients aged 6 months and older with severe hepatic impairment (Child-Pugh Class C): one tablet or one packet of oral granules once daily or less frequently
- Not recommended in patients with hepatic impairment below 6 months of age
- CFTR gene mutations responsive to Kalydeco are marked with an asterisk
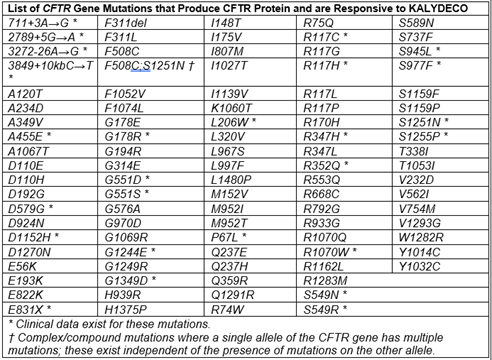
- Kalydeco should be taken with fat-containing food
- Kalydeco is not recommended for use in children aged 4 months to less than 6 months with hepatic impairment and/or taking concomitant moderate or strong CYP3A inhibitors
- Warnings for transaminase (ALT or AST) elevations, concomitant use with CYP3A inducers, cataracts
Policy Updates:
- 10/19/21 – New policy approved by P&T.
References:
- Kalydeco Prescribing Information. Vertex Pharmaceuticals Incorporated. Boston, MA. December 2020.
- Ramsey BW, Davies J, McElvaney G, et al. A CFTR potentiator in patients with cystic fibrosis and the G551D mutation. N Engl J Med. 2011;365:1663-1672.
Last review date: October 19, 2021

