Sodium Oxybate Agents for Narcolepsy (LUMRYZ, XYREM, XYWAV)
LUMRYZ (sodium oxybate) extended-release oral solution
XYREM (sodium oxybate) oral solution; Sodium Oxybate oral solution, authorized brand alternative (ABA)
XYWAV (calcium, magnesium, potassium & sodium oxybates) oral solution
Diagnosis considered for coverage:
Xyrem (sodium oxybate) oral solution; Sodium Oxybate oral solution, ABA
- Narcolepsy with Cataplexy (i.e., Narcolepsy Type 1): Indicated for the treatment of cataplexy in patients 7 years of age and older with narcolepsy.
- Narcolepsy without Cataplexy (i.e., Narcolepsy Type 2): Indicated for the treatment of excessive daytime sleepiness (EDS) in patients 7 years of age and older with narcolepsy.
Xywav (calcium, magnesium, potassium, and sodium oxybates) oral solution
- Narcolepsy with Cataplexy (i.e., Narcolepsy Type 1): Indicated for the treatment of cataplexy in patients 7 years of age and older with narcolepsy.
- Narcolepsy without Cataplexy (i.e., Narcolepsy Type 2): Indicated for the treatment of excessive daytime sleepiness (EDS) in patients 7 years of age and older with narcolepsy.
- Idiopathic Hypersomnia (IH): Indicated for the treatment of idiopathic hypersomnia in adults.
Lumryz (sodium oxybate) extended - release oral solution
- Narcolepsy with Cataplexy (i.e., Narcolepsy Type 1): Indicated for the treatment of cataplexy in adults with narcolepsy.
- Narcolepsy without Cataplexy (i.e., Narcolepsy Type 2): Indicated for the treatment of excessive daytime sleepiness (EDS) in adults with narcolepsy.
Coverage Criteria:
For diagnosis of narcolepsy with cataplexy (i.e., Narcolepsy Type 1):
- Diagnosis of narcolepsy as confirmed by sleep study (unless the prescriber provides justification confirming that a sleep study would not be feasible); AND
- Symptoms of cataplexy are present; AND
- Symptoms of excessive daytime sleepiness (e.g., irrepressible need to sleep or daytime lapses into sleep) are present; AND
- Prescribed by or in consultation with one of the following:
- Neurologist
- Psychiatrist
- Sleep Medicine Specialist; AND
- For Lumryz only: Trial and failure, contraindication or intolerance to TWO of the following:
- Xyrem ABA by Hikma
- Xywav
- Wakix
- For Xyrem and Xyrem ABA by Amneal only: Trial and failure, contraindication or intolerance to the following:
- Xyrem ABA by Hikma
For diagnosis of narcolepsy without cataplexy (i.e., Narcolepsy Type 2):
- Diagnosis of narcolepsy as confirmed by sleep study (unless the prescriber provides justification confirming that a sleep study would not be feasible); AND
- Symptoms of cataplexy are absent; AND
- Symptoms of excessive daytime sleepiness (e.g., irrepressible need to sleep or daytime lapses into sleep) are present; AND
- Trial and failure, contraindication (e.g., safety concerns, not indicated for patient's age/weight), or intolerance to BOTH of the following:
- generic modafinil or generic armodafinil
- Sunosi; AND
- One of the following:
- Trial and failure, contraindication, or intolerance to an amphetamine (e.g., amphetamine, dextroamphetamine) or methylphenidate-based stimulant
- History of or potential for a substance use disorder; AND
- Prescribed by or in consultation with one of the following:
- Neurologist
- Psychiatrist
- Sleep Medicine Specialist; AND
- For Lumryz only: Trial and failure, contraindication (e.g., safety concerns, not indicated for patient's age/weight) or intolerance to ONE of the following:
- Xyrem ABA by Hikma
- Xywav
- Wakix
- For Xyrem and Xyrem ABA by Amneal only: Trial and failure, contraindication or intolerance to the following:
- Xyrem ABA by Hikma
For diagnosis of idiopathic hypersomnia (IH) (Xywav only):
- Diagnosis of idiopathic hypersomnia as confirmed by sleep study (unless the prescriber provides justification confirming that a sleep study would not be feasible); AND
- Symptoms of excessive daytime sleepiness (e.g., nap duration of longer than 60 minutes) are present; AND
- Prescribed by or in consultation with one of the following:
- Neurologist
- Psychiatrist
- Sleep Medicine Specialist
Reauthorization Criteria:
For diagnosis of narcolepsy with cataplexy (i.e., Narcolepsy Type 1):
- One of the following
- Documentation demonstrating a reduction in the frequency of cataplexy attacks associated with therapy.
- Documentation demonstrating a reduction in symptoms of excessive daytime sleepiness associated with therapy.
For diagnosis of narcolepsy without cataplexy (i.e., Narcolepsy Type 2):
- Documentation demonstrating a reduction in symptoms of excessive daytime sleepiness associated with therapy.
For diagnosis of IH (Xywav only):
- Documentation demonstrating a reduction in symptoms of excessive daytime sleepiness associated with therapy.
Dosing:
- Xyrem; Sodium Oxybate oral solution, ABA; Xywav:
- Narcolepsy with or without cataplexy (adults)
- Initiate dosage at 4.5 g per night orally, divided into two doses.
- Titrate to effect in increments of 1.5 g per night at weekly intervals (0.75 g at bedtime and 0.75 g taken 2.5 to 4 hours later).
- Recommended dosage range: 6 g to 9 g per night orally.
- Initiate dosage at 4.5 g per night orally, divided into two doses.
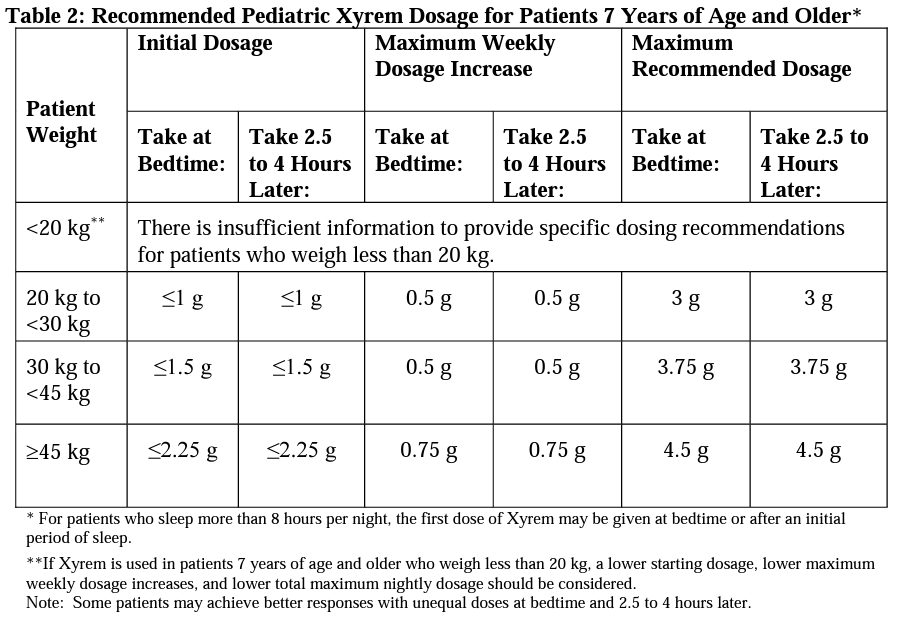
- Narcolepsy with or without cataplexy (pediatrics, 7 years of age and older)
- Idiopathic Hypersomnia (Xywav only) (adults)
- Twice nightly regimen: Initiate dosage at 4.5 g or less per night orally, divided into two doses. Titrate to effect in increments of up to 1.5 g per night per week, up to 9 g total nightly dose.
- Once nightly regimen: Initiate dosage at 3 g or less per night orally, as one dose. Titrate to effect in increments of up to 1.5 g per night per week, up to 6 g total nightly dose.
- Narcolepsy with or without cataplexy (adults)
- Lumryz:
- Narcolepsy with or without cataplexy (adults)
- Initiate dosage at 4.5 g per night orally.
- Titrate to effect in increments of 1.5 g per night at weekly intervals.
- Recommended dosage range: 6 g to 9 g once per night orally.
- Narcolepsy with or without cataplexy (adults)
Coverage Duration:
- Initial: 6 months
- Reauthorization: 1 year
Authorization is not covered for the following:
- The following conditions and other uses of this drug for indications not listed in this policy do not meet the coverage criteria established by the Western Health Advantage (WHA) Pharmacy and Therapeutics Committee.
- Concomitant use of Xyrem, Xywav, or Lumryz with other sodium oxybate-based agents, pitolisant (Wakix) and/or solriamfetol (Sunosi).
Additional Information:
- Narcolepsy is a chronic neurological disorder of hypersomnia and its associated symptoms are potentially debilitating. Narcolepsy is typically classified as type 1 (narcolepsy with cataplexy, NT1) or type 2 (narcolepsy without cataplexy, NT2)
- Cataplexy is a sudden loss of muscle tone while a person is awake leads to weakness and a loss of voluntary muscle control.
- Excessive daytime sleepiness (EDS) is present in all patients with narcolepsy. Other symptoms include cataplexy, hypnagogic hallucinations, and sleep paralysis; however, only about one-third of patients have all 4 symptoms.
- Objective measures of EDS as assessed by sleep latency (ie, the time interval between attempting to fall asleep and the onset of sleep) measured using polysomnography (PSG) include the Maintenance of Wakefulness Test (MWT) and Multiple Sleep Latency Test (MSLT)
- Subjective measures of EDS include Epworth Sleepiness Scale (ESS), Clinical Global Impression of Change (CGI-C), Clinical Global Impression of Improvement (CGI-I), and Patient Global Impression of Change (PGI-C)
- Idiopathic hypersomnia (IH) is a rare sleep disorder that is characterized by chronic EDS and often difficulty waking up from nocturnal sleep or daytime naps. The condition is categorized as a central disorder of hypersomnolence. The pathophysiology of IH is not well understood, and diagnosis requires exclusion of other more common causes of excessive sleepiness.
- International classification of Sleep Disorders (ICSD-3) diagnostic criteria for narcolepsy with cataplexy (narcolepsy type 1):
- Daily periods of irrepressible need for sleep or daytime lapses into sleep (i.e., excessive daytime sleepiness) for at least 3 months.
- Presence of one or both of the following: cataplexy and a mean sleep latency of less than or equal to 8 minutes and 2 or more sleep onset REM periods (SOREMPs) on a multiple sleep latency test (MSLT) performed using standard techniques (a SOREMP within 15 minutes of sleep onset on the preceding nocturnal polysomnogram may replace 1 of the SOREMPs on the MSLT); or cerebrospinal fluid (CSF) hypocretin-1 concentration is less than or equal to 110 pg/mL or less than one-third of the mean values obtained in normal subjects with the same standardized assay).
- Exclusion of alternative causes of chronic daytime sleepiness by history, physical exam, and polysomnography. Other conditions that cause chronic daytime sleepiness include insufficient sleep, untreated sleep apnea, periodic limb movements of sleep, and idiopathic hypersomnia (chronic sleepiness but without SOREMPs or other evidence of abnormal REM sleep). In addition, the effects of sedating medications should be excluded.
- ICSD-3 diagnostic criteria for narcolepsy without cataplexy (narcolepsy type 2):
- Daily periods of irrepressible need for sleep or daytime lapses into sleep (i.e., excessive daytime sleepiness) for at least 3 months.
- Cataplexy is absent
- CSF hypocretin-1 levels, if measured, is greater than 110 pg/mL or greater than one-third of the mean values obtained in normal subjects with the same standardized assay)
- A mean sleep latency of less than or equal to 8 minutes and 2 or more SOREMPs on a MSLT performed using standard techniques (a SOREMP within 15 minutes of sleep onset on the preceding nocturnal polysomnogram may replace 1 of the SOREMPs on the MSLT).
- Exclusion of alternative causes of chronic daytime sleepiness by history, physical exam, and polysomnography. Other conditions that cause chronic daytime sleepiness include insufficient sleep, untreated sleep apnea, periodic limb movements of sleep, and idiopathic hypersomnia (chronic sleepiness but without SOREMPs or other evidence of abnormal REM sleep). In addition, the effects of sedating medications should be excluded.
- ICSD-3 diagnostic criteria for idiopathic hypersomnia requires all of the following:
- Daily periods of irrepressible need for sleep or daytime lapses into sleep (i.e., excessive daytime sleepiness) for at least 3 months
- Cataplexy is absent
- A MSLT documents fewer than two SOREMPs, or no SOREMPs if the REM sleep latency on the preceding polysomnogram was ≤15 minutes
- The presence of at least one of the following: MSLT shows a mean sleep latency of ≤8 minutes or Total 24-hour sleep time is ≥660 minutes (typically 12 to 14 hours) on 24-hour polysomnography or by wrist actigraphy in association with a sleep log
- Insufficient sleep syndrome is ruled out (if deemed necessary, by lack of improvement of sleepiness after an adequate trial of increased nocturnal time in bed, preferably confirmed by at least a week of wrist actigraphy) 6. No better explanation by another sleep disorder, medical or psychiatric disorder or use of drugs or medications.
Policy Updates:
- 10/17/2017– New policy for Xyrem approved by WHA P&T Committee.
- 09/03/2021 – New policy for Xywav approved by WHA P&T Committee and criteria update for Xyrem
- 02/15/2022 – Criteria approved for Xywav for treatment of idiopathic hypersomnia.
- 11/14/2023 – Add Lumryz to existing Xyrem and Xywav program. Update program title: Sodium Oxybate Agents for Narcolepsy (LUMRYZ, XYREM, XYWAV). Remove requirement for antidepressants for NT1. Remove requirement for modafinil and methylphenidate for IH. Remove reference to dose, age, chart notes, and concomitant use from coverage criteria and manage these aspects through other policy sections (e.g., indication, dosing, etc.). (P&T 11/14/23)
- 3/1/2024 (policy effective date)- Update PA to require step through Xyrem ABA Hikma for Xyrem by Amneal, Xyrem ABA by Amneal, and Lumryz (P&T 2/20/2024) (P&T meeting February)
References:
- Xyrem Prescribing Information. Jazz Pharmaceuticals, Inc. Palo Alto, CA. December 2020.
- Morgenthaler TI, Kapur VK, Brown T, et al. Practice parameters for the treatment of narcolepsy and other hypersomnias of central origin: An American Academy of Sleep Medicine report. Sleep. 2007 Dec;30(12):1705-11.
- Wise MS, Arand DL, Auger RR, et al. Treatment of narcolepsy and other hypersomnias of central origin: An American Academy of Sleep Medicine review. Sleep. 2007 Dec;30(12):1712-27.
- International classification of sleep disorders. 3rd ed. Darien, IL: American Academy of Sleep Medicine; 2014.
- Sateia MJ. International classification of sleep disorders - third edition: highlights and modifications. CHEST. 2014 Nov;146(5):1387-1394.
- Sun, Eric. Narcolepsy. 2021 Feb. Available at https://www.sleepfoundation.org/narcolepsy. Accessed October 21, 2021.
- Franceschini, C., Pizza, F., et al. A practical guide to the pharmacological and behavioral therapy of Narcolepsy. Neurotherapeutics volume 18, pages 6–19 (2021). Available at https://link.springer.com/article/10.1007/s13311-021-01051-4. Accessed October 21, 2021.
- Maski, K., Trotti, L., et al. Treatment of central disorders of hypersomnolence: an American Academy of Sleep Medicine systematic review, meta-analysis, and GRADE assessment. J Clin Sleep Med. 2021;17(9):1895–1945. Available at https://jcsm.aasm.org/doi/10.5664/jcsm.9326. Accessed October 21, 2021.
- Sodium Oxybate Prescribing Information. Hikma Pharmaceuticals, USA Inc. Berkeley Heights, NJ. October 2022.
- Lumryz Prescribing Information. Avadel CNS Pharmaceuticals, LLC. Chesterfield, MO. May 2023.
Last review date: March 1, 2024