Weight Loss Medications
phentermine, ADIPEX-P (phentermine), LOMAIRA (phentermine), QSYMIA (topiramate-phentermine), CONTRAVE (naltrexone-bupropion), SAXENDA (liraglutide), WEGOVY (semaglutide), ZEPBOUND (tirzepatide)
Diagnosis considered for coverage:
- Adipex-P (phentermine), Lomaira (phentermine) - short-term (a few weeks) adjunct in a regimen of weight reduction based on exercise, behavioral modification and caloric restriction in the management of exogenous obesity.
- Contrave - indicated as an adjunct to a reduced-calorie diet and increased physical activity for chronic weight management in adults.
- Qsymia, Saxenda, Wegovy - indicated as an adjunct to a reduced-calorie diet and increased physical activity for chronic weight management in patients aged12 years and older.
- Zepbound - indicated as an adjunct to a reduced-calorie diet and increased physical activity for chronic weight management in adults.
Coverage Criteria:
For treatment of weight management:
- Dose does not exceed FDA label maximum (see below); AND
- Adipex-P (phentermine) – up to 37.5 mg per day (1 tablet or capsule per day)
- Lomaira (phentermine)—up to 24 mg per day (1 tablet, three times daily)
- Qsymia – up to 15 mg/92 mg per day (1 capsule per day)
- Contrave – up to 32 mg/360 mg per day (4 tablets per day)
- Saxenda – up to 3 mg subcutaneous injection once daily (15 ml per 30 days)
- Wegovy – up to 2.4 mg subcutaneous injection once weekly (3 ml per 28 days)
- Zepbound—up to 15 mg injected subcutaneously once weekly; AND
- One of the following:
- For Qsymia, Saxenda, or Wegovy: Patient is 12 years of age or older
- For Contrave, phentermine, or Zepbound: Patient is 18 years of age or older; AND
- Patient has a current BMI that corresponds to 30 kg/m2 or greater for adults by international cut-offs (Cole Criteria); AND
- The patient’s current weight (prior to starting treatment) is documented; AND
- Medical records AND prescription claim data confirm the patient is being treated for one or more of the following weight-related comorbidities:
- High blood pressure (hypertension)
- Type 2 diabetes mellitus
- High cholesterol (hyperlipidemia);
- Sleep apnea (verified by a sleep study); AND
- Used as an adjunct to lifestyle modification (e.g., dietary or caloric restriction, exercise, behavioral support, community-based program); AND
- Patient will not use the requested drug in combination with other agents for weight loss or glucagon-like peptide 1 (GLP-1) receptor agonists; AND
- For Saxenda, Wegovy, or Zepbound only: One of the following:
- Request for Saxenda or Wegovy: Patient is between 12 and 17 years of age
- Medical records confirm trial and failure (i.e., failure to lose weight while on therapy), clinically significant side effects with one of the following agents, or contraindication to all:
- generic phentermine
- Qsymia
- Contrave
Reauthorization Criteria:
For treatment of weight management:
- Dose does not exceed FDA label maximum (see below):
- Adipex-P (phentermine) – up to 37.5 mg per day (1 tablet or capsule, once daily)
- Lomaira (phentermine)—up to 24 mg per day (1 tablet, three times daily)
- Qsymia – up to 15 mg/92 mg per day (1 capsule, once daily)
- Contrave – up to 16 mg/180 mg per day (2 tablets, twice daily)
- Saxenda – up to 3 mg subcutaneous injection once daily (15 ml per 30 days)
- Wegovy – up to 2.4 mg subcutaneous injection once weekly (3 ml per 28 days)
- Zepbound—up to 15 mg injected subcutaneously once weekly; AND
- Patient will not use the requested drug in combination with other agents for weight loss or glucagon-like peptide 1 (GLP-1) receptor agonists; AND
- The patient’s current weight and BMI is documented; AND
- Patient has achieved and maintained greater than 5% weight loss after starting treatment.
Coverage Duration:
- Initial and Reauthorization: 6 months
Authorization is not covered for the following:
The following conditions, and other uses of this drug for indications not listed in this policy, do not meet the coverage criteria established by the Western Health Advantage (WHA) Pharmacy and Therapeutics Committee.
- Type 2 diabetes mellitus
Additional Information:
- BMI is calculated by dividing weight in kilograms by height in meters squared (kg/m2).
- Examples of weight loss drugs include phentermine (Adipex-P, Lomaira), liraglutide (Saxenda), phentermine/topiramate (Qsymia), naltrexone/bupropion (Contrave), lorcaserin (Belviq), semaglutide (Wegovy), tirzepatide (Zepbound)
- Examples of GLP-1 receptor agonists include dulaglutide (Trulicity), exenatide (Byetta), extended-release exenatide (Bydureon, Bydureon BCise), liraglutide (Victoza), lixisenatide (Adlyxin, Soliqua), semaglutide (Ozempic, Rybelsus, Wegovy), tirzepatide (Mounjaro)
- Saxenda, Wegovy, and Zepbound are contraindicated in patients with a personal or family history of medullary thyroid carcinoma (MTC) and in patients with Multiple Endocrine Neoplasia syndrome type 2 (MEN 2).
- International Obesity Task Force BMI Cut-offs for Obesity by Sex and Age for Pediatric Patients Aged 12 Years and Older (Cole Criteria)
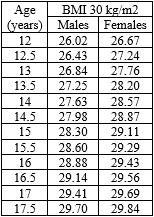
Policy Updates:
- 02/15/2022 – Weight loss agents combined into a single policy; updated indication of Saxenda to include pediatric patients age 12 years and older and added BMI conversion (Cole Criteria); updated medical necessity criteria to require active treatment of comorbid conditions; added exclusion for use in combination of GLP-1 drugs with other GLP-1 drugs; updated initial authorization period from 12 weeks to 6 months; added requirement to received documented weight prior to starting treatment.
- 05/22/2023 – Addition of sleep apnea weight-related comorbidity.
- 10/31/2023 - Update section, Diagnosis considered for coverage.
- 12/18/2023 - Addition of Zepbound and Lomaira to weight loss criteria.
References:
- Adipex-P Prescribing Information. Teva Pharmaceuticals USA, Inc. Parsippany, NJ . September 2020.
- Apovian CM, Aronne LJ, Bessesen DH, et al. Pharmacological Management of Obesity: An Endocrine Society Clinical Practice Guideline. J Clin Endocrinol Metab. 2015;100(2): 342-62.
- Contrave Prescribing Information. Nalpropion Pharmaceuticals, Inc. La Jolla, CA. October 2020.
- Davies M, Færch L, Jeppesen OK, et al. Semaglutide 2·4 mg once a week in adults with overweight or obesity, and type 2 diabetes (STEP 2): a randomised, double-blind, double-dummy, placebo-controlled, phase 3 trial. Lancet. 2021;397(10278):971-984. doi:10.1016/S0140-6736(21)00213-0
- Garvey WT, Mechanick JI, Brett EM, et al. American Association of Clinical Endocrinologists and American College of Endocrinology comprehensive clinical practice guidelines for medical care of patients with obesity. Endocr Pract. 2016 Jul;22(7):842-84. doi: 10.4158/EP161356.ESGL. https://www.aace.com/files/final-appendix.pdf. Accessed July 26, 2021.
- Lomaira Prescribing Information. KVK-Tech, Inc. Newtown, PA. December 2018.
- Phentermine 15 and 30 mg capsules Prescribing Information. Eon Labs, Inc. Princeton, NJ. October 2018.
- Qsymia Prescribing Information. Vivus, Inc. Campbell, CA. October 2020.
- National Institutes of Health, National Heart, Lung, and Blood Institute. Managing overweight and obesity in adults: systematic evidence review from the obesity expert panel, 2013. https://www.nhlbi.nih.gov/health-topics/managing-overweight-obesity-in-adults. Accessed July 26, 2021.
- Saxenda Prescribing Information. Novo Nordisk Inc. Plainsboro, NJ. December 2020.
- Styne DM, Arslanian SA, Connor EL,et al. Pediatric Obesity—Assessment, Treatment, and Prevention: An Endocrine Society Clinical Practice Guideline. J Clin Endocrinol Metab. 2017;102(3): 709-57.
- Rubino D, Abrahamsson N, Davies M, et al. Effect of Continued Weekly Subcutaneous Semaglutide vs Placebo on Weight Loss Maintenance in Adults With Overweight or Obesity: The STEP 4 Randomized Clinical Trial. JAMA. 2021;325(14):1414-1425. doi:10.1001/jama.2021.3224
- Wadden TA, Bailey TS, Billings LK, et al. Effect of Subcutaneous Semaglutide vs Placebo as an Adjunct to Intensive Behavioral Therapy on Body Weight in Adults With Overweight or Obesity: The STEP 3 Randomized Clinical Trial. JAMA. 2021;325(14):1403-1413. doi:10.1001/jama.2021.1831
- Wegovy Prescribing Information. Novo Nordisk Inc. Plainsboro, NJ. June 2021.
- Wilding JPH, Batterham RL, et al. Once-weekly Semaglutide in Adults with Overweight or Obesity. N Engl J Med. 2021;384(11):989. doi:10.1056/NEJMoa2032183.
- Zepbound Prescribing Information. Lilly USA, LLC Indianapolis, IN. November 2023.
Last review date: December 18, 2023

