Proprotein Convertase Subtilisin Kexin Type 9 (PCSK9) Inhibitors
REPATHA (evolocumab), PRALUENT (alirocumab)
Diagnosis considered for coverage:
- Repatha
- Atherosclerotic cardiovascular disease (ASCVD) - in adults with established cardiovascular disease (CVD) to reduce the risk of myocardial infarction, stroke, and coronary revascularization.
- Primary hyperlipidemia - an adjunct to diet, alone or in combination with other low-density lipoprotein cholesterol (LDL-C)-lowering therapies, in adults with primary hyperlipidemia, including heterozygous familial hypercholesterolemia (HeFH), to reduce LDL-C.
- Heterozygous familial hypercholesterolemia (HeFH) - as an adjunct to diet and other LDL-C-lowering therapies in pediatric patients aged 10 years and older with HeFH, to reduce LDL-C.
- Homozygous familial hypercholesterolemia (HoFH) - as an adjunct to other LDL-C-lowering therapies in adults and pediatric patients aged 10 years and older with HoFH, to reduce LDL-C.
- Praluent
- Atherosclerotic cardiovascular disease (ASCVD) - to reduce the risk of myocardial infarction, stroke, and unstable angina requiring hospitalization in adults with established cardiovascular disease.
- Primary hyperlipidemia (including HeFH) - as adjunct to diet, alone or in combination with other low-density lipoprotein cholesterol (LDL-C)-lowering therapies, in adults with primary hyperlipidemia, including heterozygous familial hypercholesterolemia (HeFH), to reduce LDL-C.
- Homozygous familial hypercholesterolemia (HoFH) - as an adjunct to other LDL-C-lowering therapies in adult patients with homozygous familial hypercholesterolemia (HoFH) to reduce LDL-C.
Coverage Criteria:
For diagnosis of established atherosclerotic cardiovascular disease (ASCVD):
- Dose does not exceed the Food and Drug Administration (FDA) labeled maximum:
- Repatha: 140 mg every 2 weeks OR 420 mg once monthly administered subcutaneously
- Praluent: 150 mg every 2 weeks OR 300 mg once monthly administered subcutaneously; AND
- Patient is 18 years of age or older; AND
- Prescribed by or in consultation with a cardiologist, endocrinologist, or lipid specialist; AND
- Medical record documentation confirms a diagnosis of established ASCVD by history of ONE of the following: acute coronary syndrome (ACS), myocardial infarction (MI), angina, coronary or other arterial revascularization, stroke, transient ischemic attack (TIA), or clinically significant coronary heart disease (CHD) diagnosed by testing (e.g., coronary angiography, treadmill stress test, stress echocardiography, or nuclear imaging); AND
- ONE of the following is confirmed by medical record documentation:
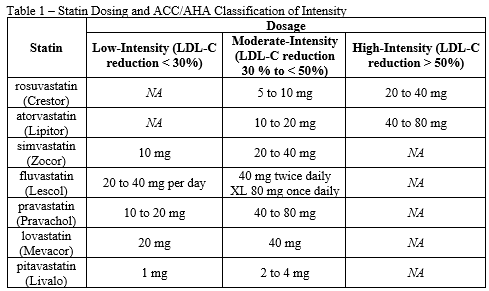
- Patient has received a high-intensity statin therapy (see Table 1) for at least 3 months and will continue to receive a high-intensity statin at maximally tolerated dose
- BOTH of the following:
- Patient experienced persistent and intolerable side effects to statin therapy (e.g. myalgia or myositis - muscle pain, weakness, or inflammation)
- ONE of the following:
- Patient has received a moderate-intensity statin therapy (see Table 1) for at least 3 months and will continue to receive a moderate-intensity statin at maximally tolerated dose
- Patient has received a low-intensity statin therapy (see Table 1) for at least 3 months and will continue to receive a low-intensity statin at maximally tolerated dose
- Patient has a contraindication to all statin therapies
- Patient has rhabdomyolysis or muscle symptoms associated with creatine kinase (CK) level increases greater than ten times the upper limit of normal (ULN); AND
- ONE of the following is confirmed by medical record documentation:
- Patient has received ezetimibe (Zetia) in combination with maximally tolerated statin therapy for at least 3 months
- If intolerant to statins, the patient has tried ezetimibe (without a statin) for at least 3 months
- Patient experienced intolerable side effects or a contraindication to ezetimibe; AND
- Patient has a low-density lipoprotein cholesterol (LDL-C) equal to or greater than 70 mg/dL while on maximally tolerated lipid-lowering therapy (e.g., statin therapy with ezetimibe) within the last 120 days.
For diagnosis of heterozygous familial hypercholesterolemia (HeFH):
- Dose does not exceed the FDA-labeled maximum:
- Repatha: 140 mg every 2 weeks OR 420 mg once monthly administered subcutaneously
- Praluent: 150 mg every 2 weeks OR 300 mg once monthly administered subcutaneously; AND
- Patient age is:
- Repatha: 10 years of age or older
- Praluent: 18 years of age or older; AND
- Prescribed by or in consultation with a cardiologist, endocrinologist, or lipid specialist; AND
- Patient has a diagnosis of HeFH that is confirmed by medical record documentation by BOTH of the following:
- Untreated/pre-treatment LDL-cholesterol (LDL-C) is greater than 190 mg/dL (greater than 155 mg/dL if less than 16 years of age)
- One of the following:
- Family history of myocardial infarction (heart attack) in first-degree relative less than 60 years of age
- Family history of myocardial infarction (heart attack) in second-degree relative less than 50 years of age
- Family history of LDL-C greater than 190 mg/dL in first- or second-degree relative
- Family history of familial hypercholesterolemia (HeFH or HoFH) in first- or second-degree relative
- Family history of tendinous xanthoma (lipid deposits in tendons) or arcus cornealis (lipid deposits in the outer part of the cornea) in first- or second-degree relative
- Patient has genetic mutation in the LDL receptor, ApoB, or PCSK9 gene
- Patient has physical signs of HeFH (i.e., tendon xanthomas, corneal arcus and age less than 45 years)
- Dutch Lipid Clinic Network diagnostic criteria for familial hypercholesterolemia score is greater than 8 (i.e., definite familial hypercholesterolemia)
- Simon Broome diagnostic criteria for familial hypercholesterolemia corresponds to definite familial hypercholesterolemia; AND
- ONE of the following is confirmed by medical record documentation:
- Patient has received a high-intensity statin therapy (see Table 1) for at least 3 months and will continue to receive a high-intensity statin at maximally tolerated dose
- BOTH of the following:
- Patient experienced persistent and intolerable side effects to statin therapy (e.g. myalgia or myositis - muscle pain, weakness, or inflammation)
- ONE of the following:
- Patient has received a moderate-intensity statin therapy (see Table 1) for at least 3 months and will continue to receive a moderate-intensity statin at maximally tolerated dose
- Patient has received a low-intensity statin therapy (see Table 1) for at least 3 months and will continue to receive a low-intensity statin at maximally tolerated dose
- Patient has a contraindication to all statin therapies
- Patient has rhabdomyolysis or muscle symptoms associated with creatine kinase (CK) level increases greater than ten times the upper limit of normal (ULN); AND
- ONE of the following is confirmed by medical record documentation:
- Patient has received ezetimibe (Zetia) in combination with maximally tolerated statin therapy for at least 3 months
- If intolerant to statins, the patient has tried ezetimibe (without a statin) for at least 3 months
- Patient experienced intolerable side effects or a contraindication to ezetimibe.
For diagnosis of homozygous familial hypercholesterolemia (HoFH):
- Dose does not exceed the FDA-labeled maximum:
- Repatha: 420 mg every 2 weeks administered subcutaneously
- Praluent: 150 mg every 2 weeks administered subcutaneously; AND
- Patient age is:
- Repatha: 10 years of age or older
- Praluent: 18 years of age or older; AND
- Prescribed by or in consultation with a cardiologist, endocrinologist, or lipid specialist; AND
- Patient has a diagnosis of HoFH that is confirmed by medical record documentation by ONE of the following:
- Patient has two genetic mutations in the LDL receptor, ApoB, PCSK9, or LDLRAP1 genes
- BOTH of the following:
- Prior to treatment, patient has an LDL-C level greater than 500 mg/dL, or the LDL-C is greater than or equal to 300 mg/dL while on lipid lowering therapy (excluding Praluent, Juxtapid, etc.)
- ONE of the following:
- Patient had cutaneous xanthomas (lipid deposits within organs that may manifest as papules, plaques, or nodules in skin) or tendon xanthomas (lipid deposits in tendons) before age 10
- Diagnosis or evidence of HeFH in both parents; AND
- PCSK9 inhibitor agent will be used in combination with a maximally tolerated statin and/or ezetimibe therapy.
Reauthorization Criteria:
Diagnosis of ASCVD, HeFH, HoFH:
- Dose does not exceed the FDA-labeled maximum for the patient’s condition; AND
- Medical records (e.g. chart notes, laboratory results) document an LDL-C reduction of 10% or more after starting PCSK9 inhibitor agent; AND
- PCSK9 inhibitor agent will continue to be used in combination with a maximally tolerated statin and/or ezetimibe therapy.
Coverage Duration:
- Initial: 6 months
- Reauthorization: 1 year
Authorization is not covered for the following:
- The use of this drug for indications not listed in this policy does not meet the coverage criteria established by the Western Health Advantage (WHA) Pharmacy and Therapeutics (P&T) Committee.
Additional Information:
- Dutch Lipid Clinic Network diagnostic criteria for Familial Hypercholesterolemia example can be accessed at http://nlaresourcecenter.lipidjournal.com/Content/PDFs/Tables/4.pdf
- Simon Broome diagnostic criteria for Familial Hypercholesterolemia example can be accessed at http://nlaresourcecenter.lipidjournal.com/Content/PDFs/Tables/3.pdf.
- Genetic testing for familial hypercholesterolemia: https://www.cdc.gov/genomics/disease/fh/testing_FH.htm
- Cutaneous xanthomas are localized lipid deposits within organs that may manifest as papules, plaques, or nodules in skin. Tendon xanthoma (also known as xanthoma tendinosum or tendinous xanthoma) are lipid deposits characterized by papules and nodules in the tendons of the hands, feet, and heel.
- First Degree Relatives include parents, siblings, and children.
- Second Degree Relatives include aunts, uncles, grandparents, grandchildren, nieces, nephews, or half-siblings.
Policy Updates:
- 07/24/2016 – Original review.
- 11/17/2020 – Annual review; updated clinical criteria.
- 02/15/2022 – Annual review; combined Repatha and Praluent policies into a single PCSK9i policy; formatting updated; reduced minimum age for Repatha for treatment of FH from age 18 to 10 based on FDA-approval; changed requirement for high potency trial from two high potency statins to a trial with one; added option to use a low to medium potency statin if high potency statin is not tolerated.
References:
- ATP III Final Report PDF. Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) Final Report. Circulation. 2002;106:3143-3421.
- Blom DJ, Hala T, Bolognese M, et al. A 52-week placebo-controlled trial of evolocumab in hyperlipidemia. N Engl J Med. 2014;370:1809-19.
- Blom DJ, Harada-Shiba M, et al. Efficacy and safety of alirocumab in adults with homozygous familial hypercholesterolemia: the ODYSSEY HoFH trial. J Am Coll Cardiol. 2020;76(2):131-142. doi:10.1016/j.jacc.2020.05.027
- Cannon CP, Blazing MA, Giugliano RP, et al. Ezetimibe added to statin therapy after acute coronary syndromes. N Engl J Med. 2015;372:2387-97.
- Cuchel M, Bruckert E, Ginsberg HN, et al. Homozygous familial hypercholesterolaemia: new insights and guidance for clinicians to improve detection and clinical management. A position paper from the Consensus Panel on Familial Hypercohlesterolaemia of the European Atherosclerosis Society. Eur Heart J. 2014;35:2146-57.
- Goldberg AC, Hopkins PN, Toth PP, et al. Familial hypercholesterolemia: screening, diagnosis, and management of pediatric and adult patients: clinical guidance from the National Lipid Association Expert Panel on Familial Hypercholesterolemia. J Clin Lipidol. 2011;5:S1-51.
- Grundy SM, Stone NJ, Bailey AL, et al. 2018 AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA Guideline on the Management of Blood Cholesterol: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J Am Coll Cardiol 2019; 73:e285-e350.
- Jellinger PS, Handelsman Y, Rosenblit PD, et al. American association of clinical endocrinologists and American college of endocrinology guidelines for management of dyslipidemia and prevention of cardiovascular disease. Endocr Pract. 2017; Suppl 2;23:1-87.
- Lloyd-Jones DM, Morris PB, Ballantyne CM, et al. 2017 Focused Update of the 2016 ACC expert consensus decision pathway on the role of non-statin therapies for LDL-cholesterol lowering in the management of atherosclerotic cardiovascular disease risk. J Am Coll Cardiol. 2017;70:1785-1822.
- Praluent Prescribing Information. Regeneron Pharmaceuticals, Inc. Tarrytown, NY. April 2021.
- Raal FJ, Honarpour N, Blom DJ, et al. Inhibition of PCSK9 with evolocumab in homozygous familial hypercholesterolaemia (TESLA Part B): a randomised, double-blind, placebo-controlled trial. Lancet. 2015;385:341-50.
- Raal FJ, Santos RD. Homozygous familial hypercholesterolemia: current perspectives on diagnosis and treatment. Atherosclerosis. 2012;223:262-8.
- Repatha [package insert]. Amgen Inc. Thousand Oaks, CA. September 2021.
- Sabatine MS, Giugliano RP, Keech AC, et al. Evolocumab and clinical outcomes in patients with cardiovascular disease. N Engl J Med. 2017;376(18):1713-22.
- Schwartz GG, Bessac L, Berdan LG, et al. Effect of alirocumab, a monoclonal antibody to PCSK9, on long-term cardiovascular outcomes following acute coronary syndromes: a rationale and design of the ODYSSEY outcomes trial. Am Heart J. 2014;168:682-9.
- Scientific Steering Committee on behalf of the Simon Broome Register Group. Risk of fatal coronary heart disease in familial hypercholesterolaemia. BMJ. 1991;303:893-6.
- The Lipid Research Clinics Coronary Primary Prevention Trial results. II. The relationship of reduction in incidence of coronary heart disease to cholesterol lowering. JAMA. 1984;251:365-74.
- WHO Familial Hypercholesterolemia Consultation Group. Familial Hypercholesterolemia (FH): report of a second WHO consultation. Geneva: World Health Organization; 1999.
Last review date: February 15, 2022

