MYRBETRIQ (mirabegron granules)
Self-Administration-Oral
Indications for Prior Authorization:
- Indicated for the treatment of neurogenic detrusor overactivity (NDO) in pediatric patients aged 3 years and older
Coverage Criteria:
For diagnosis of neurogenic detrusor overactivity:
- Dose is appropriate for the patient’s weight OR dose does not exceed 10 mL (80 mg) once daily, AND
- Patient is 3 years of age or older, AND
- Medical records document a diagnosis of neurogenic detrusor overactivity (NDO), AND
- Patient has tried and failed (a minimum 30-day supply), contraindication (e.g., safety concerns, not indicated for patient's age/weight), or intolerance to generic oxybutynin syrup or tablets, AND
- If the patient is 35 kg or more: chart notes document a medical reason why the patient cannot use Myrbetriq extended-release tablets
Reauthorization Criteria:
For diagnosis of neurogenic detrusor overactivity:
- Dose is appropriate for the patient’s weight OR dose does not exceed 10 mL (80 mg) once daily, AND
- Medical records document a positive response to therapy
Coverage Duration:
- Initial: 1 year
- Reauthorization: 1 year
Additional Information:
- Pediatric patients weighing less than 35 kg: Use Myrbetriq Granules administered once daily
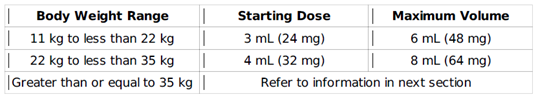
- Pediatric patients weighing 35 kg or more: Use Myrbetriq or Myrbetriq Granules
- Starting dose: 6 mL (48 mg) once daily
- Maximum dose: 10 mL (80 mg) once daily after 4 to 8 weeks
- Myrbetriq and Myrbetriq Granules are two different products and they are not substitutable on a milligram-per-milligram basis
- Do not combine Myrbetriq and Myrbetriq Granules to achieve the total dose.
- Adjust dose for patients with renal or hepatic impairment
- Warnings and precautions: increase in blood pressure, urinary retention in in patients with bladder outlet obstruction (BOO) and in patients taking muscarinic antagonist medications for the treatment of OAB, angioedema, patients taking drugs metabolized by CYP2D6
Policy Updates:
- 11/16/2021 – New policy approved by P&T
References:
- Myrbetriq Granules Prescribing Information. Astellas Pharma US, Inc. Northbrook, IL. April 2021.
Last review date: November 16, 2021

